Description
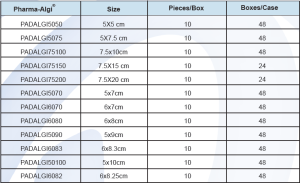
Product Info
composed of soft absorbent polyurthane foam laminated to a Fibrosol® Ag Non adhesive or Fibrosol® Ag Extra Non adhesive layer placed
as an island pad under a waterproof, virus proof and bacteria proof polyurethane film coated with hydrocolloid adhesive which turns into gel upon absorption so maintains a moist wound environment. The hydrocolloid layer is present either as a border surrounding the dressing or as a perforated layer covering the whole dressing.
• Provides excellent absorption and retention capabilities for moderate to highly exuding wounds.
• Conforms to the wound surface to form an intimate contact.
• Creates a moist environment that aids wound healing.
• Acts as a bacterial barrier that prevents outer wound infection.
• Helps reduce wound pain while the dressing is in situ and upon removal.
• Supports wound healing by providing a moist wound healing environment.
• Hemostatic effect.
• Broad antimicrobial activity.
• Infected wounds and those of risk of infection.
• Second degree burns.
• Diabetic foot ulcers, pressure ulcers and leg ulcers.
• Surgical wounds.
• Traumatic wounds.
• Wounds those are prone to bleeding.
• Oncology wounds.
• Donor and recipient graft sites.
• Treatment of primary infected wounds
• Effective bacterial barrier that reduces wound infection
• Pressure sores
• Leg ulcers
• Ischaemic and diabetic ulcers
• Fibrosol® Ag dressings should not be used on people who are allergic to any of the dressing’s components.
• Experience is limited with newly born, infants and during pregnancy.
• Fibrosol® Ag is not intended for use within internal body cavities.
• If infection develops during the use of the dressing, clinically indicated antibiotic therapy should be initiated.
• Control of blood glucose should be monitored for diabetic foot ulcers.
• Delicate newly formed blood vessels could produce blood stained wound fluid.
• Sterility is guaranteed unless pouch is damaged.






Reviews
There are no reviews yet.