Description
Product Info
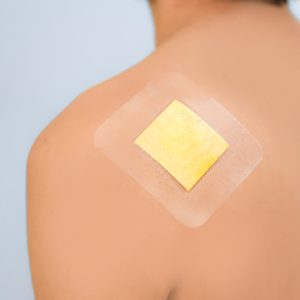
Pharmapore® IV Silver is a non woven dressing for skin sensitive people, having incision along the middle and antimicrobial absorbent silver pad at the end of Incision, located in central position.
The pad is a bilaminate construction, made of absorbent nonwoven, laminated with HDPE silver colour net. The nonwoven layer contains silver, in form of silver sodium zirconium phosphate.
Antimicrobial barrier to prevent colonization on the dressing.
Prophylaxis against infection especially for wounds at high risk of infection.
Biocompatible and complying to ISO 10993.
- Catheter and cannula fixation dressing and prophylaxis against catheter related infections (CRIs).
There are no absolute contraindications for using this product range.
Do not use for individuals with known sensitivity to any of the dressing components.
Due to presence of antimicrobial silver ions, the experience with neonates / small babies is limit
The silver ions of silver pad are non-migratory. When silver pad was tested for zone of Inhibition, there were no inhibitions of growth around the sample but only under the sample. This means that silver ions do not leach from the pad and act only at the pad surface when they come in contact with microorganisms.
1. Open the pouch using aseptic technique. In case of Pharmapore® PU I.V. Silver , hold the dressing at either
side of the wave cut and stretch the support paper a little to loosen the wave cut.
2. Remove the larger flap of the release liner to expose the adhesive surface.
3. Place the dressing on the injection site. Remove the lower flaps.
4. In case of Pharmapore® PU I.V. Silver , slowly remove the upper support paper while smoothing down the
transparent film dressing edges.
Pharmapore® I.V Silver | Size | Pieces/Case | Boxes/Case |
IVNW Ag 76 | 7 cm x 6 cm | 100 | 16 |
IVNW Ag 1012 | 10 cm x 12 cm | 100 | 16 |
IVNW Ag 68 | 6 cm x 8 cm | 100 | 16 |
Other sizes are available upon request






Reviews
There are no reviews yet.